How your Primary Practice should prepare for upcoming “Tripledemic” Vaccination Changes Implementation and Updates!
CDC's division National Center for Immunization and Respiratory Diseases (NCIRD) alongside the US Public Health Service captain recently came up with numbers and addressed how the projected increasing number of Flu and RSV cases in colder months will be dealt with this year.
After a summer of peace, it's time to gear up for the changes coming as this is the season of Vaccines and Tests. This year, the flu season might prove to be more concerning as this time COVID-19 might give it company.
Why is this season going to be hard?
- New and stronger variants are on the rise.
- Increased hospitalized cases.
- Weakened immunity in Peads and Geriatric communities.
- Timely and Easy Provision of Vaccinations.
- Now relaxed, not well-prepared population (and practices).
- COVID-19 activity makes it difficult to monitor the impact of influenza-reported stats.
There is no need to panic though, grab your favorite mug of hot cocoa and relax as we go through these updates!. In this article, we will discuss what is needed to be done to effectively order, and describe diagnosis and requirements for ultra-efficient billing and coding of much-needed tests, vaccines, and counseling. We will try to go through so you can master them before the said 'Tripledemic' hits.
Respiratory Syncytial Virus (RSV)
Other novel additions to the CPT code set respond to product-specific innovations in the prevention of Respiratory Syncytial Virus (RSV) that causes acute respiratory infection in individuals of all age groups.
Five new CPT codes have been created to report product-specific RSV immunizations (90380, 90381, 90683, 90679, and 90678) for better tracking, reporting, and analysis that supports data-driven planning and allocation.
Coronavirus SARS-CoV-2 Disease (COVID-19)
Among the important CPT changes for 2024 is the consolidation of over 50 previous codes that streamline the reporting of immunizations for COVID-19. The CPT Editorial Panel also approved the provisional codes (91318-91322) to identify monovalent vaccine products from Moderna and Pfizer for immunization against COVID-19. The provisional codes will be effective for use when the monovalent vaccine products from Moderna and Pfizer receive approval from the U.S. Food and Drug Administration. In addition, a new vaccine administration code (90480) was approved for reporting the administration of any COVID-19 vaccine for any patient, replacing all previously approved product-specific vaccine administration codes.
On Nov. 1, the American Medical Association (AMA) is scrapping almost all of the COVID-19 vaccine administration and product CPT codes it has created over the last several years. In their place, you’ll have a shorter list of product codes and a single administration code. View current COVID-19 codes at a glance on our cheat sheet.
More than 70 COVID-19 vaccine product and vaccine administration codes will be deleted effective Nov. 1, 2023. To streamline the COVID-19 immunization reporting process, the 2024 code set consolidates more than 50 previous immunization reporting codes into 17 (91300-91317), and a new code (90480) for reporting the administration of any COVID-19 vaccine for any patient. The latter replaces all previously approved specific vaccine administration codes.
New Immunization Administration for Vaccines/Toxoids Code:
Notable changes for 2024 include the consolidation of over 50 previous codes for COVID-19 immunizations, new vaccine administration codes, and five new codes for product-specific RSV immunizations. The CPT Editorial Panel has also made revisions to clarify the reporting of evaluation and management services.
New Pfizer Vaccine
Age Group: Children (6mo – 11y)
91318 – Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, diluent reconstituted, Tris-Sucrose formulation, for intramuscular use.
Dosage: 3 mcg/0.2 mL dosage – (6mo - 4y)
91319 – Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, Tris-Sucrose formulation, for intramuscular use.
Dosage: 10 mcg/0.3 mL dosage – (5y - 11y)
Age Group: 12y+ to Adults
91320 – Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, Tris-Sucrose formulation, for intramuscular use.
Dosage: 30 mcg/0.3 mL – (12y+)
New Moderna Vaccine
Age Group: Children (6mo – 11y)
91321 – Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, for intramuscular use.
Dosage: 25 mcg/0.25 mL dosage – (6mo - 11y)
Age Group: 12y+ to adults.
91322 – Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, for intramuscular use.
Dosage: 50 mcg/0.5 mL dosage – (12y+)
Revised Novavax Vaccine
Preservative free” has been removed from the code descriptor for the Novavax vaccine:
91304 – Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, recombinant spike protein nanoparticle, saponin-based adjuvant, 5 mcg/0.5 mL dosage, for intramuscular use
- This code continues to be available for use
- Also, code 90480 in addition to Administration of this vaccine.
New Immunization Administration for Vaccines/Toxoids Code
90480 - Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, single dose.
Note:
Remember: Counseling is included in code 90480 and should not be reported separately. Don’t forget to add Z23 as the associated diagnostic ICD-10 code.
Also, Worth noting Side-note: Previous Ordering Requirements have been reconciled and revised back for COVID-19 Testing in Labs.
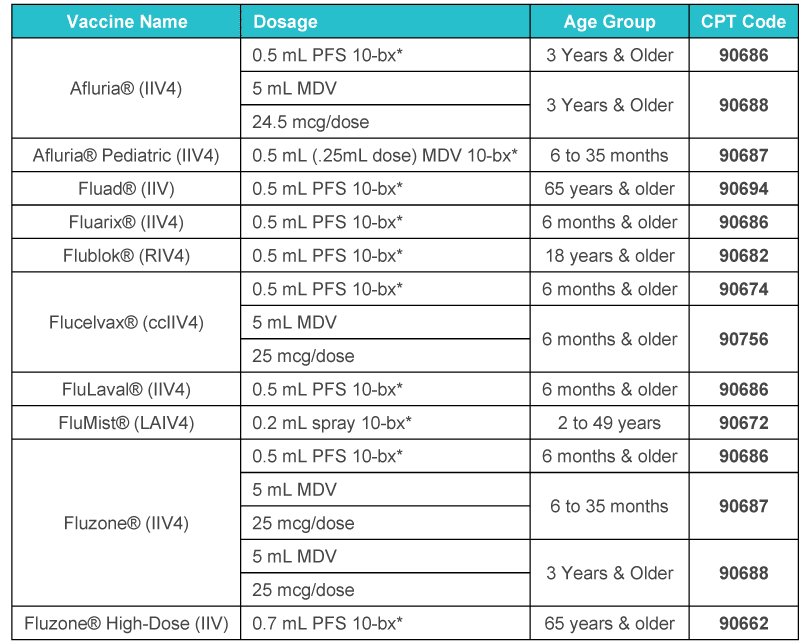
Projected U.S. Flu Vaccine Supply for the 2023-2024 Season
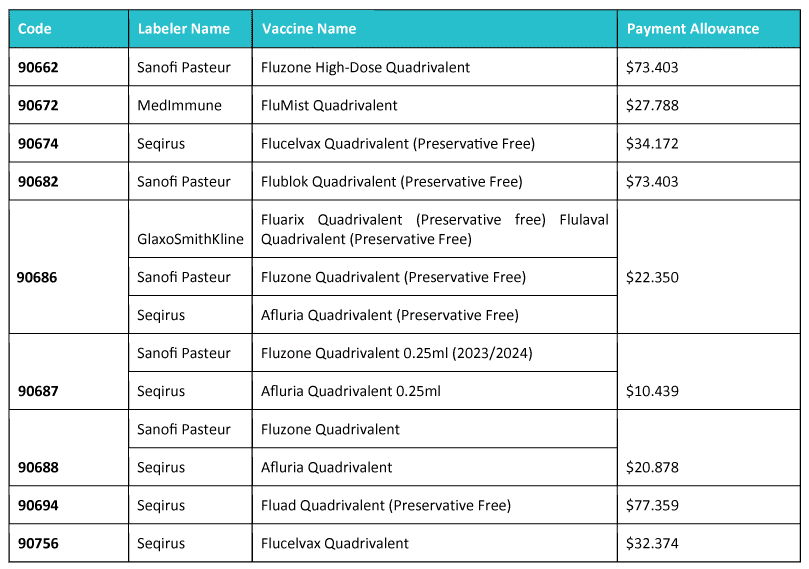
For the 2023-2024 influenza season, which runs from Aug. 1 through July 31, the Centers for Disease Control and Prevention (CDC) recommends everyone 6 months of age and older receive a flu vaccine “ideally by the end of October.” This advice has not changed from previous years, but the Medicare Part B payment allowances for the flu vaccines have changed.
Influenza A and B
Type A viruses are responsible for the highest burden of disease during seasonal epidemics, although both influenza A and B types can cause epidemics, significant diseases, and deaths.
Type B infections are less common and usually milder than influenza A (H3N2).
Vaccination is the most effective form of influenza prevention.
- Vaccine manufacturers have projected that they will supply the United States with as many as 156.2 million to 170 million doses of influenza vaccines for the 2023-2024 season. These projections may change as the season progresses.
- All flu vaccines for the 2023-2024 season will be quadrivalent (four-component).
- Most will be thimerosal-free or thimerosal-reduced vaccines (91%), and about 21% of flu vaccines will be egg-free.
- The latest information on the total distribution of influenza vaccine doses for the 2023-2024 season is available. Annual Part B deductible and coinsurance amounts do not apply for influenza virus vaccinations.
- For Medicare, report ICD-10-CM code: Z23 – Encounter for immunization on the claim and the administration code G0008 Administration of influenza virus vaccine. Unlike the vaccine product pricing, which was updated on Aug. 1, the pricing for G0008 is effective from Jan. 1 through Dec. 31.
What are Payment Allowances Changes expected this coming year?
Bottom-line:
As we go through the season that can be a troubled time for already immune-compromised or already debilitated individuals. Vaccinations in primary care can prove to be a very crucial step in your practice. Clinicians (and responsible coding practices) should carefully curate a well-thought plan in advance about eligibility and the significance of Provisions of Immunization at their Clinical Setup! Even though COVID-19 is past PHE and now may be considered a norm, doesn't mean the importance of timely vaccinations cannot be predetermined and well-managed.
Gear up your Medical Practice for the Tripledemic challenges! Stay ahead with AltuMED's expert guidance on vaccination changes, new codes, and billing strategies. Partner with us for seamless coding and billing solutions. Schedule a call now!
Subscribe to Our Newsletter!
Subscribe to Our Newsletter!
Enter Your Email Address. We Promise We Won't Spam You